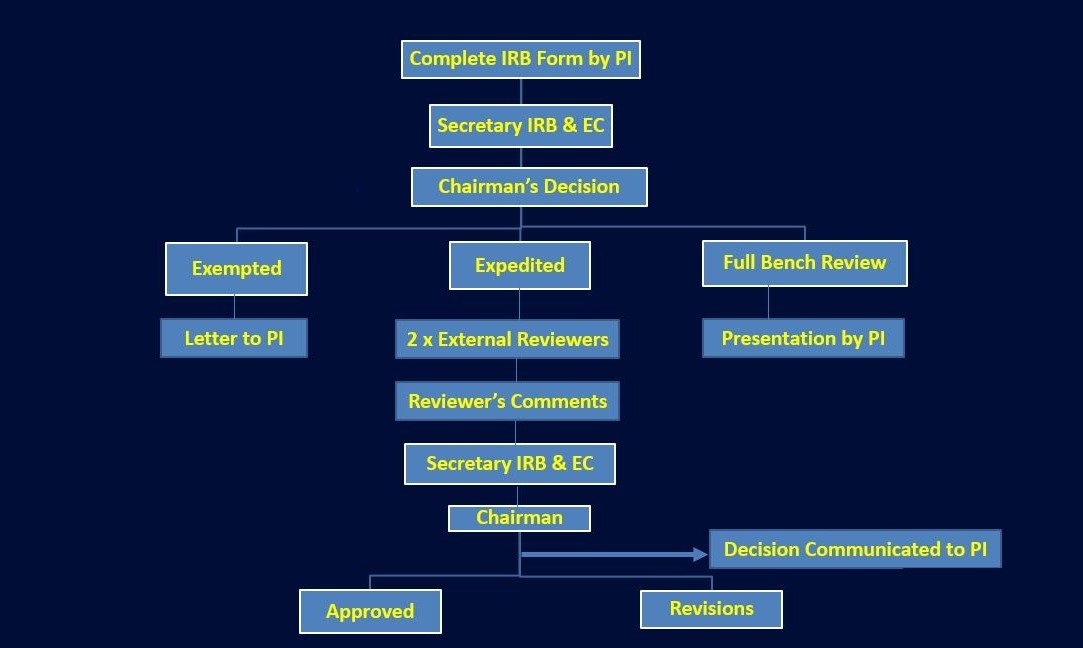
IRB & Ethical Committee is a formally designated body constituted to approve, monitor and review biomedical research involving human subjects and animals by conducting the risk-benefit analysis.
· NUMS Institutional Review Board and Ethical Committee (NUMS IRB & EC)
NUMS-Institutional Review Board & Ethical Committee (IRB & EC) has been constituted and is functional at NUMS for protecting the right and welfare of human volunteers & patients participating in a research study/clinical trial.
|
S.No |
Name/Designation |
Role |
|
a. |
Maj Gen Farrukh Saeed, HI(M) (Retd) Pro Vice Chancellor (Academics), NUMS |
Chairman |
|
b. |
Maj Gen Shazia Nisar, HI(M), HoD Medicine/ Professor of Medicine, PEMH |
Co-Chair |
|
c. |
Maj Gen Waseem Ahmad Khan, HI(M), HoD General Surgery, CMH Rwp |
Member |
|
d. |
Prof Dr. Azam Chaudhry, Dean SSH/HoD DSBS, NUMS |
Member |
|
e. |
Prof Dr. Aisha Mohy Ud Din, Dean MDS/ Dir Research, NUMS |
Member |
|
f. |
Brig Dilshad Ahmed Khan, SI(M) (Retd), Dir Academics, NUMS |
Member |
|
g. |
Brig Khadija Qamar, Professor & HoD Anatomy AM College |
Member |
|
h. |
Brig Kausar Habib (Retd), DAFNS, GHQ Rwp |
Member |
|
i. |
Brig Naubahar Hussain, SI(M) (Retd), Director Legal Affairs, NUMS |
Member |
|
j. |
Dr Shahid Waseem, Director, ORIC, NUMS |
Member |
|
i. |
Dr. Saima Pervaiz Iqbal, HoD Family Medicine STMU |
Member |
|
k. |
Col Fawad Mashhadi, Community Medicine, AM College |
Member |
|
l. |
Prof Dr. Peter John, Atta Ur Rahman School of Applied Bio Sciences (ASAB) – NUST Islamabad |
Member |
|
m. |
Associate Professor Dr. Faisal Abbas, School of Social Sciences and Humanities (S3H) - NUST Islamabad |
Member |
|
n. |
Ms. Arooj Jalil Khan, Operations Specialist, Mindray, Islamabad |
Member |
|
o. |
Lt Col Qayyum Akhter, AFID Rwp |
Member |
|
p. |
Mr. Muhammad Waheed Rashid, Lecturer Islamic Studies, NUMS |
Member |
|
q. |
Mr. Aqeel Yunus, Assistant Director, ORIC, NUMS |
Secretary |
· NUMS Institutional Animal Care & Use Committee (NUMS IACUC)
NUMS Institutional Animal Care & Use Committee (NUMS IACUC) has been constituted and is functional at NUMS for safeguarding the rights and welfare of animals utilized in research studies and clinical trials. This committee is dedicated to ensuring ethical and responsible animal research practices within the institution.
|
S.No |
Name/Designation |
Role |
|
a. |
Prof Dr Aisha Mohyuddin, Dean MDS, NUMS |
Chairperson |
|
b. |
Dr Imtiaz Ahmed Khan, Prof/Chairman, Department of Veterinary Pathology, PMAS ARID |
External Member |
|
c. |
Dr Shahid Waseem, Director ORIC, NUMS |
Member |
|
d. |
Brig Naubahar Hussain SI (M) (Retd) |
Legal Expert |
|
e. |
Dr Nazir Ahmed Lone, Associate Professor, NDBS |
Member |
|
f. |
Dr Syed Mahpara Farhat, Assistant Professor, NDBS |
Member |
|
g. |
Dr Muhammad Zeeshan Bhatti, Assistant Professor, NDBS |
Member |
|
h. |
Mr Aqeel Yunus, Assistant Director, ORIC, NUMS |
Secretary |
|
i. |
Co-opted Member (on need basis) |
- |
· NUMS Institutional Review Board and Ethical Sub-Committee (NUMS IRB & EC)
A subcommittee of NUMS IRB & Ethical Committee has been constituted and is functional at NUMS to review and approve research studies/projects of M.Phil/MS and PhD students at the National University of Medical Sciences (NUMS)
|
S.No |
Name/Designation |
Role |
|
a. |
Maj Gen Farrukh Saeed, HI(M) (Retd) Pro Vice Chancellor (Academics), NUMS |
Chairman |
|
b. |
Respective Dean |
Member |
|
c. |
Dr Shahid Waseem, Director ORIC, NUMS |
Member |
|
d. |
Subject Specialist |
Member |
|
e. |
Co-opted Member (if required) |
Member |
|
f. |
Mr Aqeel Yunus, Assistant Director, ORIC, NUMS |
Secretary |
· PEMH Institutional Review Board and Ethical Committee for Clinical Trials (PEMH IRB & EC)
An IRB & Ethical Committee has been constituted and is functional to review, monitor and approve Clinical Trials in PEMH, AFIRI, AFIMH and AFIO.
|
S.No |
Name/Designation |
Role |
|
a. |
Maj Gen Farrukh Saeed, HI(M) (Retd) Pro Vice Chancellor (Academics), NUMS |
Chairman |
|
b. |
Maj Gen Shazia Nisar, HI(M), HoD Medicine/ Professor of Medicine, PEMH |
Co-Chair |
|
c. |
Maj Gen Waseem Ahmad Khan, HI(M), HoD General Surgery, CMH Rwp |
Member |
|
d. |
Brig Naila Azam, (Retd), Professor & HoD Community Medicine,FFH |
Member |
|
e. |
Ms. Arooj Jalil Khan, Operations Specialist, Mindray, Islamabad |
Member |
|
f. |
Mrs Sobia Fatima, Advocate High Court, Awan Law Firm, Rwp |
Member |
|
g. |
Dr Nasurullah Qureshi, Lecturer Islamic Studies, NUML, Islamabad |
Member |
|
f. |
Mr Aqeel Yunus, Assistant Director, ORIC, NUMS |
Secretary |
To facilitate effective independent functioning of the IRB & Ethical Committee in NUMS and its constituent institutes/hospitals to ensure uniform ethical review process and sound scientific procedure for all research (biomedical and social science) projects involving human subjects and animal care, individuals or communities, including drug/device trial, surveys and any other research funded internationally or nationally.